Global and European Antibody Production Market Outlook 2025–2035
In terms of type, monoclonal antibody segment to command 64.0% share in the antibody production market in 2025.
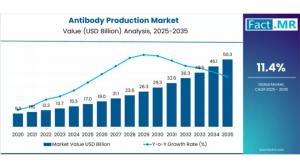
ROCKVILLE, MD, UNITED STATES, November 27, 2025 /EINPresswire.com/ -- The global antibody production market is positioned for substantial expansion as therapeutic biologics and advanced antibody modalities reshape modern medicine. Market estimates indicate growth from USD 17.03 billion in 2025 to approximately USD 50.3 billion by 2035, advancing at a CAGR of 11.4% from 2025 to 2035.Antibodies — including monoclonal antibodies, bispecific antibodies, and antibody conjugates — are increasingly central across treatment areas such as oncology, autoimmune disorders, infectious diseases, and rare genetic conditions, fueling production demand at commercial and clinical scale.
To access the complete data tables and in-depth insights, request a Discount On The Report here: https://www.factmr.com/connectus/sample?flag=S&rep_id=11974
Key Market Drivers
Therapeutic Antibody Pipelines Expanding Rapidly
Antibody-based drugs continue to lead biopharmaceutical innovation due to their clinical effectiveness and broad disease applicability. Intensified global investment in biologic R&D is accelerating demand for expanded antibody manufacturing capacity.
Outsourcing and Contract Manufacturing Growth
Biotech and pharmaceutical developers are increasingly adopting outsourcing models to access GMP-grade manufacturing, rapid scalability, and cost-efficient production environments, strengthening the role of contract manufacturers across the sector.
Rising Global Disease Burden
The increasing prevalence of cancer, immune-mediated disorders, and chronic illnesses is driving adoption of targeted antibody therapies, which directly elevates the need for higher production output and manufacturing infrastructure expansion.
Bioprocessing and Automation Advancements
Ongoing improvements in upstream cell culture, bioreactor technologies, purification systems, chromatography, filtration, single-use production components, automation, and quality analytics are enhancing yield, reducing contamination risk, and enabling commercial-grade antibody scaling.
Emerging Region Manufacturing Expansion
Production capacity is broadening beyond traditional antibody manufacturing strongholds, with emerging markets accelerating investments in biotech facility development, skilled workforce training, and biologic supply chain participation.
Market Segmentation Insights
By Product Type
Consumables and reagents represent the largest recurring revenue category due to continuous demand in R&D, clinical trials, and commercial production cycles.
Manufacturing equipment and production systems continue expanding as facilities scale capacity to meet increased biologic demand and regulatory compliance needs.
By Antibody Type
Monoclonal antibodies (mAbs) remain the most widely produced category, driven by long-established therapeutic success and mature regulatory pathways.
Bispecific antibodies and antibody-drug conjugates (ADCs) are gaining significant momentum, increasing complexity requirements for antibody production platforms.
By Process Stage
Downstream processing — including purification, polishing, validation, sterility assurance, filtration, and quality control — is a key market growth contributor due to stringent regulatory purity thresholds and evolving antibody formats.
By End-Use
Biotechnology and pharmaceutical companies are the largest consumers of antibody production services and systems.
CDMOs and CRO-driven production models are expanding rapidly as outsourcing scales globally to meet antibody pipeline manufacturing needs.
Market Challenges
Despite strong growth momentum, antibody production faces the following constraints:
High capital investment requirements for building and validating GMP-level biologic production facilities
Manufacturing complexity increases with next-generation antibody formats
Global supply chain scalability limitations in emerging regions
Need for highly skilled biologics production, cell line development, and purification experts
Regulatory and quality standardization pressures across new facilities
Competitive Landscape
The market remains driven by innovation, bioprocessing yield improvements, contract manufacturing partnerships, and strategic manufacturing capacity expansion. Competitive advantage is increasingly defined by:
Investment in scalable, contamination-resistant production platforms
Supply chain transparency and sourcing traceability
Enhanced purification and downstream processing capabilities
Deployment of automated and single-use biologic manufacturing systems
Strong partnerships between biologic developers and manufacturing providers
Purchase Full Report for Detailed Insights
For access to full forecasts, regional breakouts, company share analysis, and emerging trend assessments, you can purchase the complete report here: https://www.factmr.com/checkout/11974
Future Outlook
By 2035, antibody production is expected to become one of the most critical global biopharma infrastructure pillars. The industry is shifting toward a distributed model of biologic production hubs, flexible contract manufacturing environments, and high-yield processing ecosystems to meet next-generation therapeutic needs.
Have a specific Requirements and Need Assistant on Report Pricing or Limited Budget please contact us – sales@factmr.com
Check out More Related Studies Published by Fact.MR Research:
Blood Based Biomarkers Market - https://www.factmr.com/report/blood-based-biomarkers-market
Sports Medicine Market - https://www.factmr.com/report/sports-medicine-market
Biomarker Market - https://www.factmr.com/report/biomarkers-market
Biobanks Market - https://www.factmr.com/report/biobanks-market
About Fact.MR
Fact.MR is a global market research and consulting firm, trusted by Fortune 500 companies and emerging businesses for reliable insights and strategic intelligence. With a presence across the U.S., UK, India, and Dubai, we deliver data-driven research and tailored consulting solutions across 30+ industries and 1,000+ markets. Backed by deep expertise and advanced analytics, Fact.MR helps organizations uncover opportunities, reduce risks, and make informed decisions for sustainable growth.
S. N. Jha
Fact.MR
+1 628-251-1583
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.